Chromatography – a modern method for analyzing complex mixtures
One of the widely used methods in modern analytical chemistry is chromatography, which serves with high precision in separating complex chemical mixtures into individual components and identifying specific substances within their composition. Chromatography is a physicochemical method of separating a mixture into its components by passing it through a stationary phase (a liquid or solid absorbent).Depending on the type of mobile phase, chromatography methods can vary. If the mobile phase is a liquid or gas, the method is referred to as liquid chromatography or gas chromatography, respectively. Gas chromatography, in turn, is one of the most widely used and effective methods for analyzing complex organic mixtures today.
What is the basis of gas chromatography?
In the absorption form of gas chromatography, the mixture to be separated is passed through a layer of a specific absorbent. The separation process is based on the varying degrees of absorption (adsorption) of each component of the mixture by the absorbent under specific conditions. In other words, because each component of the mixture has a different absorption coefficient, they pass through and are retained by the absorbent at different times and to different extents, resulting in their separation.In the type known as gas-liquid chromatography, an inert gas flow serves as the mobile phase, interacting with a liquid stationary phase. In this method, a solid phase coated with liquid retains the components of the mixture and differentiates their movement, facilitating their separation.
Capillary chromatography
In capillary chromatography, the stationary phase is applied to the inner wall of an extremely thin capillary (0.25–0.35 mm in diameter). In this case, the components of the mixture are separated based on their differing solubility in the liquid stationary phase or the stability of the complexes they form. As a result, each component exits at different time intervals, allowing for precise identification.
The importance of chromatography
The primary goal of chromatography is to separate the components of a mixture. However, this method is used not only for separation but also for detecting the presence or quantity of specific components in the analyzed mixture. Gas chromatography is widely applied for this purpose across various fields, including chemistry, medicine, ecology, pharmaceuticals, and the food industry.
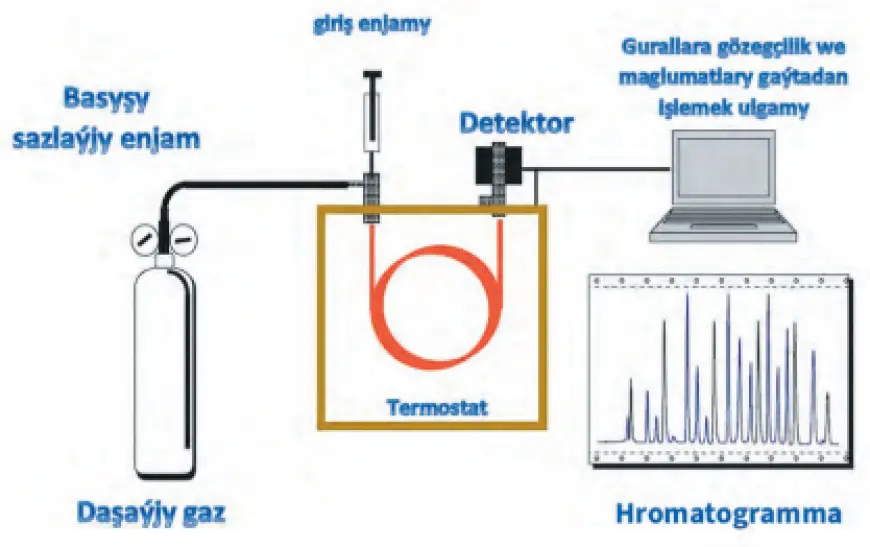
Structure and operation of gas chromatography
In gas chromatography, the mixture to be analyzed is introduced directly or through a specialized bypass chamber into a separation column. The mixture passes through the column in a flow of inert gas. The column’s structure can vary—it may consist of a solid absorbent or a solid inert substrate impregnated with a liquid. Some components pass through the column without being retained, while others are fully absorbed or distributed between the stationary phase and the gas carrier. This process enables their separation. However, in some cases, the separation characteristics of certain components may overlap, causing them to exit together, which can complicate the analysis.
Instrumentation and detectors
Various technical instruments and detectors are used in gas chromatography. These devices often operate at temperatures up to 350°C and analyze the composition of both gaseous and liquid components. The separation process can occur under isothermal (constant temperature) or programmed temperature conditions. To detect the components as they exit, various high-precision detectors are used, such as flame ionization detectors, thermal conductivity detectors (katharometers), and mass spectrometers.
Chromatography, with its refined theory and modern techniques, is one of the fundamental pillars of analytical chemistry today. Despite its complexity, its capabilities are vast. It delivers remarkable results in organic chemistry, pharmaceutical production, quality control of food products, detection of environmentally harmful substances, and scientific research.